Arm yourself with knowledge. This is the greatest thing that you can do for your health.
While the modern diet has brought with it a great deal of convenience, the mass production of readily accessible food has also brought with it an era of health decline and rampant tooth decay. In order to maximize your and your children’s dental health, it takes an understanding of the structures and their breakdown and regeneration. Here we go…..
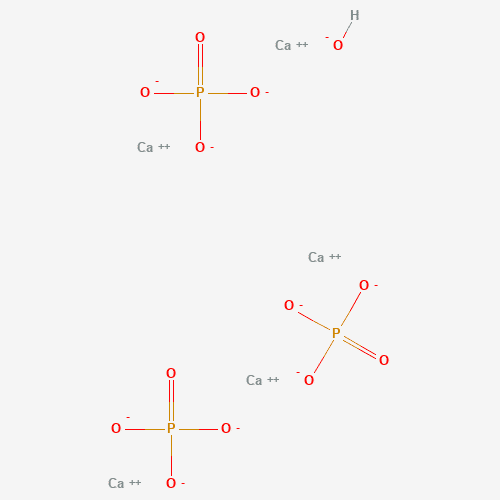
The mineral backbone of our teeth is Hydroxyapatite, making up 97% of our tooth enamel. The dominating mineral components of Hydroxyapatite are Calcium and Phosphorus.
Maintaining dental health requires a homeostasis of mineral density in tooth structure. This means a balance of Remineralization and Demineralization. When this mineral is extensively stripped from our teeth without sufficient replenishment, we end up with tooth decay and/or erosion away of tooth structure.
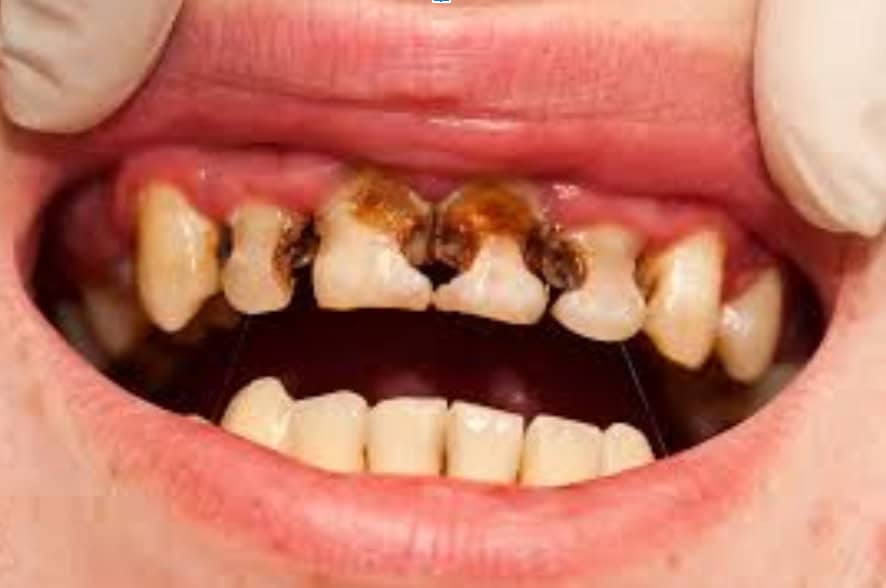
Quick pause to understand the anatomy of a tooth.
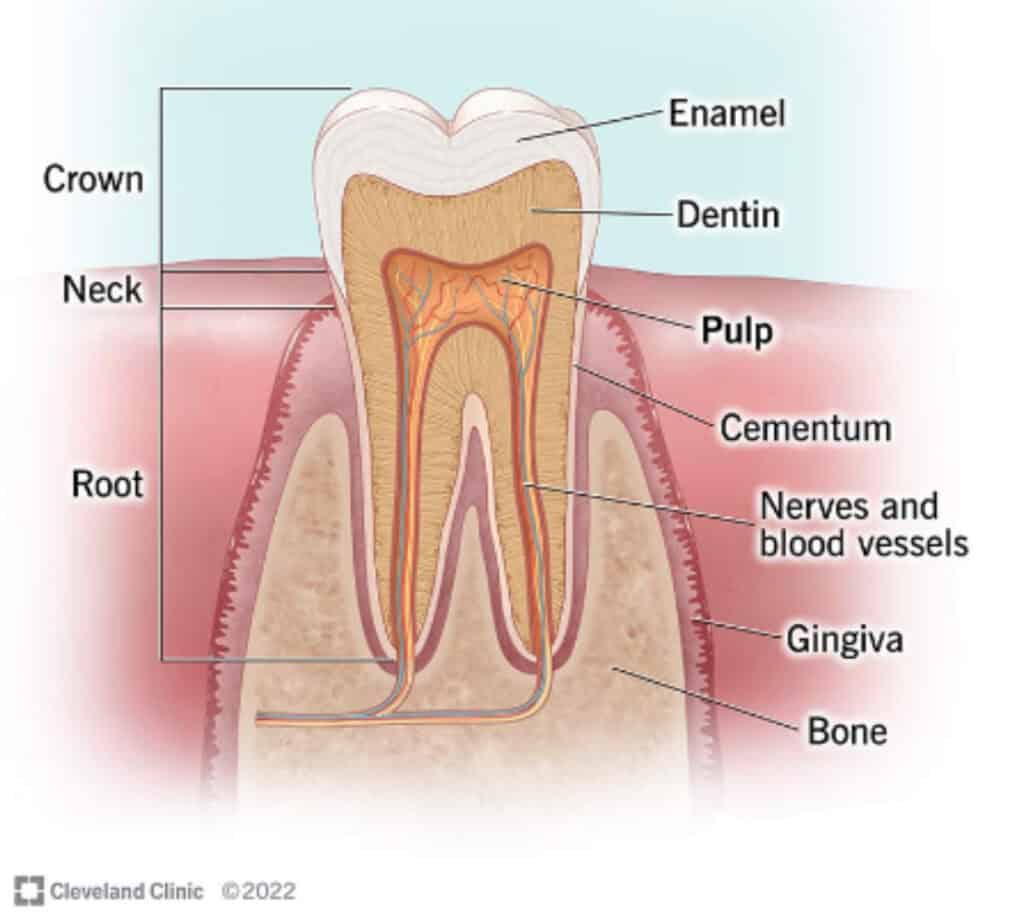
The outer layer of our tooth, the enamel, is the strongest and most mineralized layer. Enamel is thickest and strongest at the chewing surface of the tooth and gets thinner and more porous (weaker) as it tapers to the root by the gumline. The middle layer of the tooth is dentin, which is less mineralized and more porous, with the porosity getting greater as you get deeper into the dentin. Understandably, this layer is the more sensitive layer of the tooth. These pores are in direct communication with the pulp, the innermost layer of the tooth. The pulp has no mineral content. It is a soft tissue layer that contains blood vessels, the nerve of the tooth, and lymphatic tissue. The outer portion of the pulp contains construction cells called odontoblasts. These cells are essentially the handyman of the tooth that can, with sufficient mineral building blocks, lay down reparative and reactionary dentin in response to pathogens, irritants, and injury.
When dental caries (tooth decay) is in the strong enamel outer layer, it is “incipient” and reversible. Maximizing topical remineralization and minimizing demineralization can heal it. If a cavity reaches the dentin layer, it is no longer reversible. If confined to the outer third of dentin, it could potentially be arrested (stop the growth), but this will take a VERY disciplined approach to shift the demineralization/remineralization balance. If the dental caries reaches past the outer third, into the more porous part of the dentin layer, effects on the dental pulp are greater with higher risk of irreversible damage to the pulp, which can ultimately lead to an infected tooth. Restoration is necessary and immediate improvement of the demineralization/remineralization balance is critical..
In the Battle of Demineralization and Remineralization, We Must Assure that Remineralization Wins!!
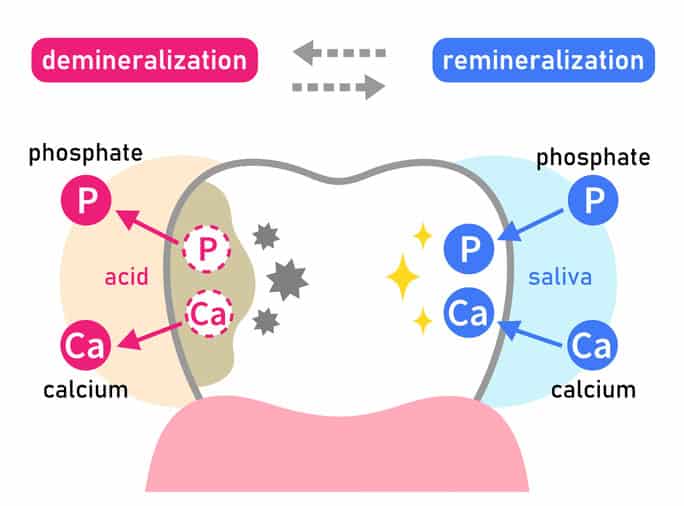
What Demineralizes Our Teeth??
In acidic environment strips the Hydroxyapatite mineral from our teeth, making the surface weak and more porous. Nearly every time we eat or drink anything (unless plain water), our mouth becomes acidic.
The oral bacteria Strep Mutans is the main decay-causing bacteria in our mouth, rapidly digesting any sugar and simple starch in our diet to acid. Be mindful of what you and your child are eating and drinking with any frequency. Strep Mutans is going to be fat and happy with frequency of any sugar, sucrose or fructose. That being said, clinical observation and research has shown that high fructose corn syrup (HFCS) causes a greater and more rapid decrease in oral pH to the acidic state. While neither are good, HFCS is like adding diesel to a fire. Also, please understand that fructose is the sugar in fruit so, when you drink fruit juice, you have removed all of the slow digesting fiber from the fruit and just have a glass full of the worst and most rapid pathogen feasting part- fructose!! Frequent fruit juice is a BIG cause of tooth decay! Simple starch (think white flour in crackers and other quick grab snacks) also is very rapidly digested by oral pathogens to acid that in turn demineralizes teeth.
Sugar and starch don’t have to be present to demineralize teeth. Any acidity in what we eat or drink erodes and demineralizes tooth enamel. Diet pop, flavored water, coffee, lemon in water… prolonged acidic state in the mouth from these acidic beverages can and will erode away enamel.
Tips to reduce demineralization:
- Limit Frequency and Length of exposure.
- Don’t sip on a sugary or acidic beverage over a long time, which would keep your mouth in a long state of low acidic pH. Drink it fast. Drink it through a straw to reduce tooth exposure.
- The worst snacks have simple sugar or simple starch that get stuck in your teeth. Crackers and gummy snacks are big criminals in the fight of tooth decay, as exposure time is large with the extended time they spend stuck in and on the teeth..
- The worst offenders in food and beverage have simple sugar (cane sugar or, even worse, high fructose), simple starch, high acidity, and tendency to coat or get stuck in and between teeth.

- Less offensive, but still demineralizing are those that may advertise “sugar-free” or “organic” or “full of vitamins”, but are still acidic and coat/stick to teeth.

What Remineralizes Our Teeth??
SALIVA!! It’s free and it’s constant, so arguably the best remineralizing agent for our teeth! Our saliva contains the calcium and phosphate necessary to rebuild Hydroxyapatite stripped from the enamel surface. If our oral microbiome is in good balance, the healthy bacteria in our biofilm actually help facilitate this process. If we have been feeding the bad Strep Mutans to frequently with simple sugars and starches, this necessary balance will not be present.
Remineralization from inside the teeth. The most important factor in internal tooth mineralization is a nutrient dense diet! If our levels of Vitamins D3 and K2 are sufficient, then odontoblasts inside the pulp tissue of our teeth will have the calcium and phosphorus building blocks they need to build and lay down reactionary and reparative dentin tooth structure to protect the pulp of the tooth from injury.
Topical remineralization. This applies to anything providing surface minerals to be absorbed into the demineralized porous enamel to rebuild and repair the damage.

HYDROXYAPATITE. Hands down the best surface remineralizing agent available. Oral products that contain it are the gold standard. The best ingredient to replace a missing ingredient is that ingredient itself. Hydroxyapatite is missing, we replace it with Hydroxyapatite. Make sure your daily toothpaste has Hydroxyapatite in it. Tinctures, mints, and chewing gum are now available with Hydroxyapatite, as well. It’s natural, it’s safe, it’s effective.You can find some examples on the Recommended Products page of our site. Some points on absorption and particle size…
- NANO Hydroxyapatite will better absorb into the surface of teeth to remineralize
- Non-Nano will not absorb as well, so it’s not as efficient at mineralizing teeth
- Key point: You want NANO particles if you want them to absorb (as in Hydroxyapatite in toothpaste). You want NON-Nano particles if you DON’T want them to absorb (as in Zinc Oxide in sunscreen).


What about Fluoride??
Topical fluoride can make the Hydroxyapatite structure in enamel more resistant to acid breakdown but ONLY when indicated and when used strategically, minimally, and responsibly.
With controlled topical application of medically compounded products to teeth, fluoride can be incorporated into the Hydroxyapatite (HAP) tooth matrix, replacing a hydroxyl group (OH), to create Fluorohydroxyapatite (F-HAP). If we have uncontrolled demineralization and/or dental decay, responsible and topical use of fluoride can provide some additional protection to the tooth matrix. Controlled and responsible.
- F-HAP is more resistant to the stripping demineralization in an acidic oral environment.
- For active tooth decay and uncontrolled pH balance of the mouth with predominant demineralization, a very controlled and strategic topical use of medically compounded sodium fluoride (NaF) can help to tip the scales to remineralization.
- Too frequent fluoride exposure, particularly during tooth development, can go too far and replace all of the OH groups in HAP to create Fluorapatite which is brittle and more at risk of fracture, as it does not have the flexure of natural Hydroxyapatite or Fluorohydroxyapatite.

“The dose makes the poison”. Controlled and responsible. Controlled and responsible. Controlled and responsible. I know that I sound like a broken record, but it is important to recognize the potential topical benefit in appropriate situations while understanding the risk of systemic toxicity. There is a good reason why a tube of fluoride toothpaste says to contact the poison control center immediately if ingested.


Free Fluorine is the most reactive chemical element on the periodic table. Fluoride should NEVER be systematically ingested. This means that it should never be added to drinking water. The fluoride that is added to drinking water is an industrial waste product, Fluorosilicic Acid. If you live in an area with fluoridated water, I would highly recommend a filtration system in your home. We have the Radiant Life filtration system in our home.
Understand the systemic toxicity of fluoride and you’ll understand why its use should only be warranted, topical, infrequent, and controlled. Again, fluoride should never be ingested.
Ingested Fluoride is a neurotoxin. Ingested Fluoride outcompetes Iodine in the Thyroid. Ingested Fluoride binds into bone HAP matrix and makes bones brittle (skeletal fluorosis). Inhaled fluorine gas severely damages the lungs.
Topical fluoride should only be used with decay risk, with active decay, or with imbalance of demineralization/remineralization. It should not be used daily. Your daily toothpaste should contain Hydroxyapatite. If active decay or high risk, you can occasionally and strategically use a toothpaste with topical fluoride. If risk of decay, consider an infrequent (twice a year) professional application of a medically compounded sodium fluoride. Controlled and strategic use. Not blanket use for everyone with a pulse.
For my fellow health-conscious followers, let’s put this into perspective. “The dose makes the poison”. We routinely use Ozone in our office as an adjunct for treatment of periodontal disease, decay, pulpal infection, alveolar infection, etc. Ozone is a highly effective and holistic adjunct to these treatments of various oral infections. Ozone insufflation can also be very effective for treatment of paranasal sinus and other craniofacial infections. HOWEVER, the proper concentration and the proper delivery of this powerful gas is critical. It can be toxic to lungs and blood cells with improper use, so strategic and appropriate use is critical. The people who promote systemic fluoride in the drinking water are often the same who would condemn ozone use as toxic. This is because they don’t understand the properties or indications for either of them. Let’s educate ourselves and use available resources appropriately and to our benefit, never to our detriment.
If you are interested in more reading on the history of water fluoridation, some great books are The Case Against Fluoride by Paul Connett, and The Fluoride Deception by Christopher Bryson. These provide insight on how industrial need and regulatory capture/confusion took something that had potential to be helpful in certain circumstances if used minimally and appropriately and turned it into the introduction of a toxin into the population’s drinking water supply.
Always in pursuit of Truth and Living Our Best Life,
✌️ ~Dr. Mindy
